Introducing the Moles Grams and Molecules Worksheet, an invaluable resource designed to illuminate the intricacies of these fundamental chemistry concepts. This worksheet empowers students with the knowledge and skills to navigate the complexities of chemical reactions and delve into the fascinating world of stoichiometry.
Through a series of engaging activities and exercises, this worksheet guides learners through the conversion between moles, grams, and molecules, unraveling the significance of Avogadro’s number. It delves into the calculation of percent composition, the determination of empirical and molecular formulas, and the identification of limiting reactants.
Introduction
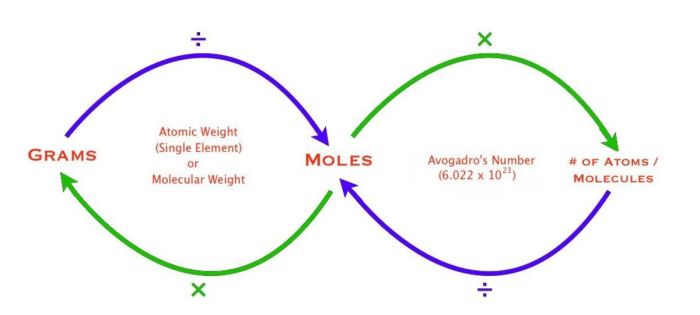
In chemistry, the concepts of moles, grams, and molecules are fundamental to understanding the composition and behavior of matter. These three units of measurement provide a framework for quantifying the amount of substance present in a sample and understanding the relationships between different chemical species.
A mole is the SI unit of amount of substance. It is defined as the amount of substance that contains exactly 6.02214076×10^23 elementary entities. These entities can be atoms, molecules, ions, electrons, or other specified particles. The mole concept allows chemists to express the quantity of a substance in terms of a specific number of particles, providing a convenient and standardized way to compare the amounts of different substances.
The mass of a substance is expressed in grams. One gram is defined as one-thousandth of a kilogram. The mass of a substance is directly proportional to the number of moles present. The molar mass of a substance is the mass of one mole of that substance.
It is expressed in grams per mole (g/mol) and provides a conversion factor between the mass and the amount of substance.
A molecule is the smallest unit of a compound that retains the chemical properties of that compound. Molecules are composed of atoms that are held together by chemical bonds. The number of molecules in a sample is directly proportional to the number of moles present.
Avogadro’s number (6.02214076×10^23) represents the number of molecules in one mole of any substance.
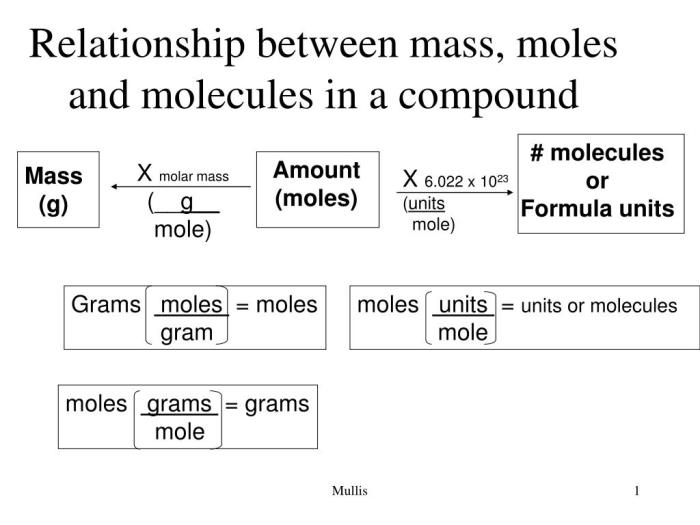
Relationship between Moles, Grams, and Molecules
The relationship between moles, grams, and molecules can be summarized by the following equation:
Number of moles = Mass (in grams) / Molar mass
This equation allows chemists to convert between the different units of measurement. For example, if we know the mass of a substance in grams and its molar mass, we can calculate the number of moles present. Conversely, if we know the number of moles and the molar mass, we can calculate the mass of the substance in grams.
Examples of Moles, Grams, and Molecules in Everyday Life
- The amount of caffeine in a cup of coffee is often expressed in milligrams. However, chemists may use moles to express the amount of caffeine more precisely.
- The concentration of a chemical solution is often expressed in molarity, which is the number of moles of solute per liter of solution.
- The number of molecules in a breath of air can be estimated using Avogadro’s number.
Converting Between Moles, Grams, and Molecules
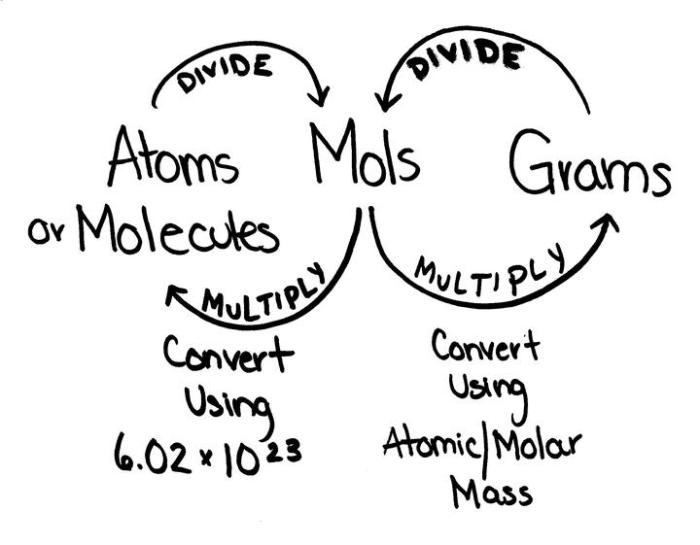
Converting between moles, grams, and molecules is essential for understanding chemical reactions and stoichiometry. These conversions involve using the mole, which is the SI unit for the amount of substance, and Avogadro’s number, which is the number of particles (atoms, molecules, or ions) in one mole of a substance.
Converting Between Moles and Grams
To convert between moles and grams, use the molar mass of the substance. Molar mass is the mass of one mole of a substance and is expressed in grams per mole (g/mol).Formula:“`Grams = Moles × Molar massMoles = Grams / Molar mass“`Example:Convert 2.5 moles of NaCl to grams.Molar
mass of NaCl = 58.44 g/molGrams = 2.5 moles × 58.44 g/mol = 146.1 g
Converting Between Moles and Molecules
To convert between moles and molecules, use Avogadro’s number (6.022 × 10^23 molecules/mol).Formula:“`Molecules = Moles × Avogadro’s numberMoles = Molecules / Avogadro’s number“`Example:Convert 3.0 × 10^24 molecules of H2O to moles.Molecules = 3.0 × 10^24 moleculesMoles = 3.0 × 10^24 molecules / 6.022 × 10^23 molecules/mol = 5.0 moles
Converting Between Grams and Molecules
To convert between grams and molecules, combine the formulas for converting between moles and grams and moles and molecules.Formula:“`Molecules = Grams × (Avogadro’s number / Molar mass)Grams = Molecules × (Molar mass / Avogadro’s number)“`Example:Convert 25.0 g of CO2 to molecules.Molar
mass of CO2 = 44.01 g/molMolecules = 25.0 g × (6.022 × 10^23 molecules/mol / 44.01 g/mol) = 3.51 × 10^23 molecules
Avogadro’s Number
Avogadro’s number is a fundamental constant in chemistry that represents the number of atoms or molecules present in one mole of a substance. It is defined as 6.02214076×10 23and is named after the Italian scientist Amedeo Avogadro.
The significance of Avogadro’s number lies in its ability to establish a bridge between the macroscopic and microscopic scales in chemistry. It allows us to convert between the amount of a substance in grams (mass) and the number of individual atoms or molecules present in that sample.
Calculating Molar Mass, Moles grams and molecules worksheet
To determine the molar mass of a substance, we can use the following formula:
Molar Mass = Mass of Substance (in grams) / Number of Moles
For example, if we have 10 grams of sodium chloride (NaCl), we can calculate its molar mass as follows:
- Mass of NaCl = 10 grams
- Number of Moles of NaCl = Mass / Molar Mass
- Molar Mass of NaCl = 58.44 grams/mole
- Number of Moles of NaCl = 10 grams / 58.44 grams/mole = 0.171 moles
Calculating Number of Molecules
To determine the number of molecules present in a given sample, we can use the following formula:
Number of Molecules = Number of Moles × Avogadro’s Number
For example, if we have 0.171 moles of NaCl, we can calculate the number of molecules present as follows:
- Number of Moles of NaCl = 0.171 moles
- Number of Molecules of NaCl = Number of Moles × Avogadro’s Number
- Number of Molecules of NaCl = 0.171 moles × 6.02214076×10 23molecules/mole = 1.031×10 23molecules
Percent Composition
Percent composition is a measure of the relative amount of each element in a compound. It is expressed as a percentage and is calculated by dividing the mass of each element in the compound by the total mass of the compound and multiplying by 100%.
Example
Consider a compound with a mass of 100 grams, containing 40 grams of carbon and 60 grams of oxygen. The percent composition of this compound would be:Percent Composition of Carbon = (Mass of Carbon / Total Mass) x 100%= (40 g / 100 g) x 100%= 40%Percent Composition of Oxygen = (Mass of Oxygen / Total Mass) x 100%= (60 g / 100 g) x 100%= 60%
Empirical and Molecular Formulas
Chemical formulas provide information about the elemental composition of a compound. Empirical formulas give the simplest whole-number ratio of elements in a compound, while molecular formulas indicate the actual number of atoms of each element in a molecule of the compound.
Determining Empirical Formulas
To determine the empirical formula of a compound, we need to know the mass of each element present. This information can be obtained from elemental analysis, which involves various techniques to determine the elemental composition of a compound.
Once we have the masses of each element, we convert them to moles using their respective atomic masses. The mole ratio of the elements is then simplified to the smallest whole numbers, giving us the empirical formula.
Determining Molecular Formulas
The molecular formula of a compound is a multiple of its empirical formula. To determine the molecular formula, we need to know the empirical formula and the molar mass of the compound. The molar mass can be determined experimentally using various methods, such as mass spectrometry or combustion analysis.
We then divide the molar mass by the empirical formula mass to get the molecular formula multiple. The molecular formula is obtained by multiplying the empirical formula by this multiple.
Example
Consider a compound with the following elemental composition: 40.0% carbon, 6.7% hydrogen, and 53.3% oxygen. The molar mass of the compound is determined to be 60.0 g/mol.
Empirical Formula:
- Convert mass percentages to grams: 40.0 g C, 6.7 g H, 53.3 g O
- Convert grams to moles: 3.33 mol C, 6.7 mol H, 3.33 mol O
- Simplify mole ratio: C 1H 2O 1(empirical formula)
Molecular Formula:
- Molecular formula multiple: 60.0 g/mol (molar mass) / 30.0 g/mol (empirical formula mass) = 2
- Molecular formula: C 2H 4O 2
Limiting Reactants
In a chemical reaction, a limiting reactant is the reactant that is completely consumed, thereby limiting the amount of product that can be formed. The other reactants in the reaction are present in excess, meaning they are not completely consumed and some of them remain after the reaction is complete.
To determine the limiting reactant in a reaction, we need to compare the moles of each reactant to the stoichiometry of the reaction. The stoichiometry of a reaction is the ratio of the moles of each reactant to the moles of each product, as given by the balanced chemical equation.
Calculating the Limiting Reactant
To calculate the limiting reactant, we can use the following steps:
- Calculate the moles of each reactant using the given mass and molar mass.
- Divide the moles of each reactant by its stoichiometric coefficient in the balanced chemical equation.
- The reactant with the smallest mole ratio is the limiting reactant.
For example, consider the reaction between hydrogen and oxygen to form water:
“`
H2+ O 2→ 2H 2O
“`
If we have 2 moles of hydrogen and 1 mole of oxygen, we can calculate the mole ratio as follows:
“`Mole ratio of H 2: 2 moles / 2 = 1Mole ratio of O 2: 1 mole / 1 = 1“`
Since the mole ratio of oxygen is smaller, oxygen is the limiting reactant.
7. Stoichiometry: Moles Grams And Molecules Worksheet
Stoichiometry is the study of the quantitative relationships between the reactants and products in a chemical reaction. It helps us to predict the amount of reactants and products that are involved in a given reaction.To use stoichiometry to solve problems, we need to use the balanced chemical equation for the reaction.
The balanced chemical equation tells us the mole ratios of the reactants and products. We can use these mole ratios to convert between the masses of the reactants and products.For example, let’s say we want to know how many grams of sodium chloride (NaCl) are produced when 25.0 g of sodium (Na) reacts with excess chlorine gas (Cl 2). The balanced chemical equation for this reaction is:“`
Na + Cl2→ 2 NaCl
“`This equation tells us that 2 moles of Na react with 1 mole of Cl 2to produce 2 moles of NaCl. We can use this mole ratio to convert between the mass of Na and the mass of NaCl.First, we need to convert the mass of Na to moles:“`
0 g Na × (1 mol Na / 22.99 g Na) = 1.09 mol Na
“`Then, we can use the mole ratio to convert the moles of Na to the moles of NaCl:“`
09 mol Na × (2 mol NaCl / 2 mol Na) = 1.09 mol NaCl
“`Finally, we can convert the moles of NaCl to grams:“`
09 mol NaCl × (58.44 g NaCl / 1 mol NaCl) = 63.6 g NaCl
“`Therefore, 25.0 g of Na will react with excess Cl 2to produce 63.6 g of NaCl.
Top FAQs
What is the relationship between moles, grams, and molecules?
Moles represent the amount of substance, grams represent mass, and molecules represent the number of individual particles. Avogadro’s number provides the conversion factor between moles and molecules.
How do I determine the limiting reactant in a reaction?
The limiting reactant is the reactant that is completely consumed in a reaction, limiting the amount of product that can be formed. To identify the limiting reactant, compare the mole ratios of the reactants to their stoichiometric coefficients.