Kinetic theory of gases worksheets offer a comprehensive exploration of the fundamental principles governing the behavior of gases. These worksheets delve into the basic concepts, assumptions, and applications of the kinetic theory, providing a solid foundation for understanding the properties and interactions of gases in various real-world scenarios.
By delving into the relationship between pressure, volume, temperature, and the number of gas molecules, students gain a deeper comprehension of the molecular-level interactions that shape the macroscopic properties of gases. The worksheets also explore how the kinetic theory can be applied to optimize industrial processes, understand atmospheric phenomena, and advance scientific research.
1. Kinetic Theory of Gases: Kinetic Theory Of Gases Worksheets
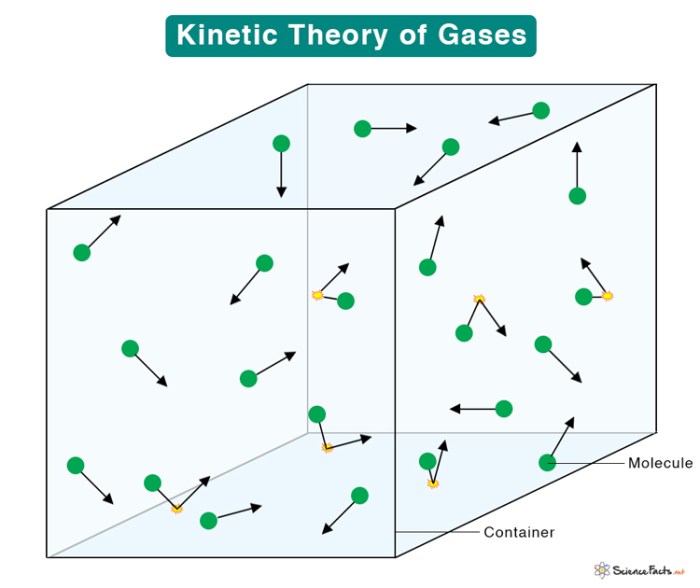
The kinetic theory of gases is a model that describes the behavior of gases based on the motion of their constituent molecules. It assumes that gas molecules are tiny, spherical particles that are in constant random motion. These molecules collide with each other and with the walls of their container, exchanging momentum and energy.
The kinetic theory of gases can be used to explain a wide range of phenomena, including the pressure, volume, and temperature of gases. It can also be used to predict the behavior of gases in various situations, such as when they are compressed or heated.
1.1 Basic Concepts and Assumptions
- Gases consist of tiny, spherical particles called molecules.
- Gas molecules are in constant random motion.
- Gas molecules collide with each other and with the walls of their container.
- The average kinetic energy of gas molecules is proportional to the absolute temperature of the gas.
1.2 Relationship between Pressure, Volume, Temperature, and Number of Molecules
The pressure, volume, temperature, and number of molecules in a gas are related by the ideal gas law:
PV = nRT
where:
- P is the pressure of the gas
- V is the volume of the gas
- n is the number of molecules in the gas
- R is the ideal gas constant
- T is the absolute temperature of the gas
1.3 Examples of Applications
- The kinetic theory of gases can be used to explain why gases expand when heated.
- The kinetic theory of gases can be used to predict the rate of effusion of a gas.
- The kinetic theory of gases can be used to design and optimize industrial processes that involve gases.
2. Applications of the Kinetic Theory of Gases
The kinetic theory of gases has a wide range of applications in science and engineering. It is used to design and optimize industrial processes, to understand atmospheric phenomena, and to advance scientific research.
2.1 Industrial Applications
- The kinetic theory of gases is used to design and optimize processes such as combustion, refrigeration, and air conditioning.
- The kinetic theory of gases is used to design and optimize the flow of gases in pipelines and other industrial equipment.
2.2 Atmospheric Phenomena
- The kinetic theory of gases is used to explain phenomena such as the wind, the formation of clouds, and the behavior of the atmosphere.
- The kinetic theory of gases is used to predict the behavior of gases in the atmosphere, such as their density and temperature.
2.3 Scientific Research
- The kinetic theory of gases is used to study the behavior of gases in extreme conditions, such as high temperatures and pressures.
- The kinetic theory of gases is used to develop new theories and models for the behavior of gases.
3. Experiments and Demonstrations
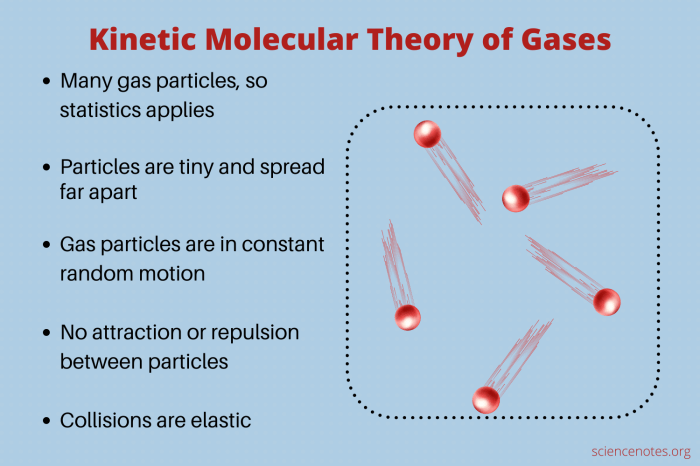
There are a number of experiments and demonstrations that can be used to demonstrate the kinetic theory of gases. These experiments and demonstrations can be used to teach students about the basic concepts of the kinetic theory of gases and to show them how the kinetic theory of gases can be used to explain real-world phenomena.
3.1 Experiment to Demonstrate the Kinetic Theory of Gases
One simple experiment that can be used to demonstrate the kinetic theory of gases is the Brownian motion experiment. This experiment involves observing the motion of small particles suspended in a fluid. The particles will move in a random, zigzag pattern due to the collisions with the molecules of the fluid.
3.2 Everyday Materials Demonstration, Kinetic theory of gases worksheets
Another simple demonstration of the kinetic theory of gases can be created using a balloon. If you fill a balloon with air and then release it, the balloon will fly around the room due to the collisions of the air molecules with the balloon.
3.3 Resources for Experiments and Demonstrations
- Science Buddies: Kinetic Theory of Gases
- Education.com: Kinetic Theory of Gases
- Khan Academy: Kinetic Theory of Gases
4. Worksheets and Activities
There are a number of worksheets and activities that can be used to help students learn about the kinetic theory of gases. These worksheets and activities can be used to reinforce the concepts of the kinetic theory of gases and to provide students with opportunities to apply their knowledge.
4.1 Worksheet to Practice Applying the Kinetic Theory of Gases
One worksheet that can be used to help students practice applying the kinetic theory of gases is the “Kinetic Theory of Gases Worksheet” from Science Buddies. This worksheet includes a number of questions that require students to use the kinetic theory of gases to explain real-world phenomena.
4.2 Activity to Explore the Kinetic Theory of Gases in a Hands-On Way
One activity that can be used to help students explore the kinetic theory of gases in a hands-on way is the “Kinetic Theory of Gases Lab” from Education.com. This activity involves students building a model of a gas and then using the model to investigate the properties of gases.
4.3 Resources for Worksheets and Activities
- Science Buddies: Kinetic Theory of Gases
- Education.com: Kinetic Theory of Gases
- Khan Academy: Kinetic Theory of Gases
General Inquiries
What is the kinetic theory of gases?
The kinetic theory of gases is a model that describes the behavior of gases based on the assumption that gas molecules are in constant random motion.
What are the basic assumptions of the kinetic theory of gases?
The basic assumptions of the kinetic theory of gases are that gas molecules are point masses, they are in constant random motion, and they do not interact with each other except through elastic collisions.
What is the relationship between pressure, volume, temperature, and the number of gas molecules?
The relationship between pressure, volume, temperature, and the number of gas molecules is described by the ideal gas law, which states that PV = nRT, where P is the pressure, V is the volume, n is the number of moles of gas, R is the gas constant, and T is the temperature.