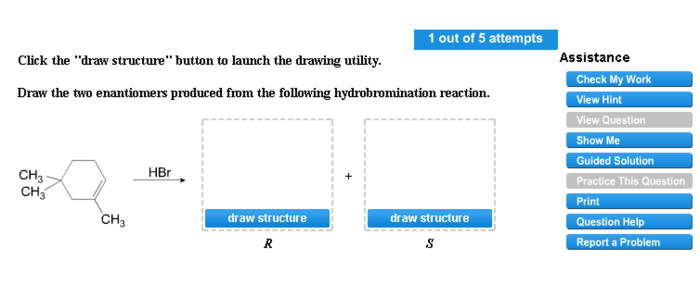
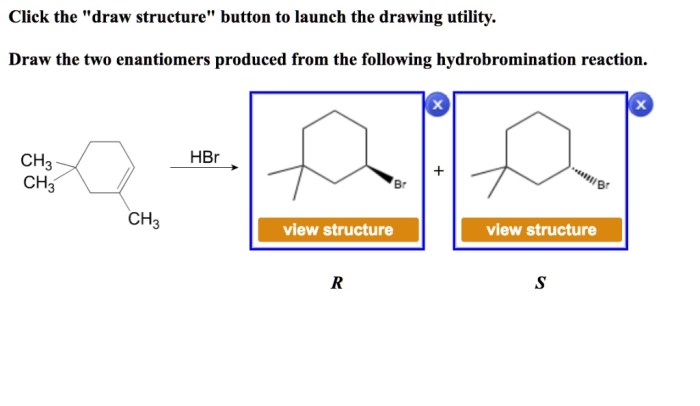
Draw the two enantiomers produced from the following hydrobromination reaction – In the realm of organic chemistry, the concept of enantiomers, mirror-image molecules that cannot be superimposed, holds immense significance. This article delves into the fascinating world of enantiomers, exploring their unique properties and the intricacies of their formation through hydrobromination reactions.
By examining the stereochemistry of these reactions, we gain insights into the factors that govern their selectivity and the applications of enantiomers in various scientific disciplines.
Enantiomers Introduction
Enantiomers are stereoisomers that are mirror images of each other and cannot be superimposed on each other. They have the same molecular formula and connectivity but differ in the spatial arrangement of their atoms.
Examples of enantiomers in everyday life include our hands, feet, and many chiral molecules such as amino acids and sugars.
Hydrobromination Reaction
Hydrobromination is an electrophilic addition reaction in which hydrogen bromide (HBr) adds to an alkene or alkyne. The electrophile in this reaction is the HBr molecule, and the nucleophile is the alkene or alkyne.
The mechanism of hydrobromination involves the following steps:
- The HBr molecule approaches the alkene or alkyne, and the electrophilic bromine atom attacks the double or triple bond.
- A new bond is formed between the bromine atom and one of the carbon atoms in the alkene or alkyne.
- The remaining hydrogen atom from the HBr molecule adds to the other carbon atom in the alkene or alkyne.
Product Analysis: Draw The Two Enantiomers Produced From The Following Hydrobromination Reaction
The hydrobromination of an alkene or alkyne can produce two enantiomers. The two enantiomers are formed because the addition of HBr to the double or triple bond can occur from either side of the molecule.
To identify the two enantiomers, we need to determine the absolute configuration of each carbon atom that is involved in the double or triple bond. The absolute configuration of a carbon atom is specified by the Cahn-Ingold-Prelog (CIP) priority rules.
Stereochemistry of the Product
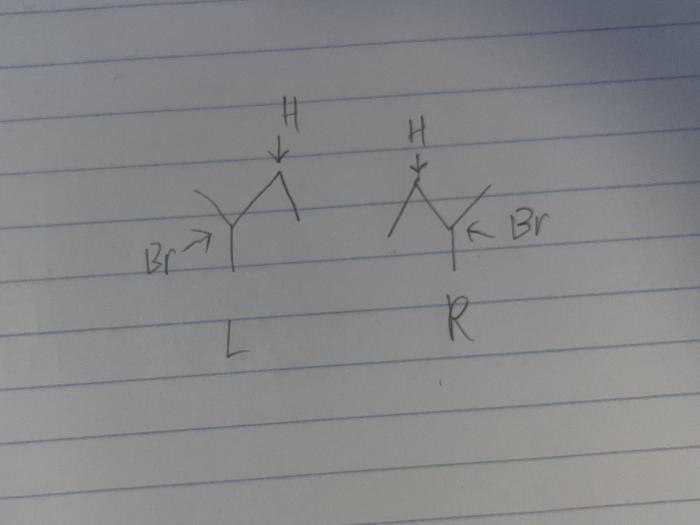
The stereochemical outcome of the hydrobromination reaction is determined by the regioselectivity and stereoselectivity of the reaction.
Regioselectivity refers to the preference for the addition of HBr to one side of the double or triple bond over the other. Stereoselectivity refers to the preference for the formation of one enantiomer over the other.
Applications of Enantiomers
Enantiomers are important in various fields, including pharmaceuticals, agrochemicals, and materials science.
In pharmaceuticals, enantiomers can have different biological activities. For example, one enantiomer of a drug may be active, while the other enantiomer is inactive or even harmful.
Common Queries
What is the difference between enantiomers and diastereomers?
Enantiomers are non-superimposable mirror images of each other, while diastereomers are stereoisomers that are not mirror images.
How can we determine the absolute configuration of enantiomers?
The absolute configuration of enantiomers can be determined using various methods, such as X-ray crystallography or NMR spectroscopy.
What factors influence the stereoselectivity of hydrobromination reactions?
The stereoselectivity of hydrobromination reactions is influenced by factors such as the nature of the alkene, the solvent, and the reaction conditions.